Introduction
Plants in nature are faced with attack by potentially several hundred thousand species of herbivorous insects. Nevertheless, the world is still green, and any given plant species is resistant to attack by most insects. To a large extent, resistance to herbivory is mediated by a wide array of toxic and deterrent plant metabolites. Between- and within-species variation in the production of defensive chemicals often determines which plants a particular insect species is able to consume. Some economically important plant toxins, e.g. nicotine in tobacco and glucosinolates in cruciferous vegetables, have been studied extensively. However, the great majority of plant defensive metabolites remain completely unknown. A typical leaf contains a few thousand different small molecules that can be detected by mass spectrometry, but only a few hundred of these have identified structures. Many, perhaps most of these completely unknown plant metabolites function in defense against herbivores and/or pathogens.
The Jander lab studies the genetic and biochemical mechanisms that mediate plant interactions with insect herbivores. This includes not only the identification of novel defense-related plant metabolites, but also characterization of the genes and enzymes that are involved in their biosynthesis. Plant species that are currently being investigated include Zea mays (maize), Arabidopsis thaliana (mouse-ear cress), Asclepias syriaca (common milkweed), Asclepias syriaca (tropical milkweed), Erysimum cheiranthoides (wormseed wallflower), and Nicotiana benthamiana (an Australian tobacco species). Genetic mapping of natural variation in insect resistance, mass spectrometry-based screens to identify previously unknown plant defensive metabolites, and characterization of biosynthetic enzymes through knockout mutations and in vitro enzyme assays have led to the discovery of novel plant defense mechanisms. On the insect side of the interaction, a major research focus is the investigation of strategies that herbivores use to avoid plant defenses or suppress them in a targeted manner.
Examples of current research projects in the Jander lab are:
Maize-insect interactions

Thirteen corn leaf aphids (Rhopalosiphum maidis) and one green peach aphid (Myzus persicae) on the stem of a maize plant.
Benzoxazinoids, a group indole-derived metabolites, have a prominent role in the herbivore defenses of maize, wheat, rye, and other grasses. Research in the Jander lab has included the discovery of previously unknown genes involved in maize benzoxazinoid biosynthesis, isolation of mutations that affect defense-induced benzoxazinoid production, and investigation of defensive trade-offs in the production of different types of benzoxazinoids. A current research focus is the regulation of other maize defenses by benzoxazinoids and their breakdown products.
Among insects that feed on maize, Rhopalosiphum maidis (corn leaf aphids) have been a longer-term research interest in the lab. Cultivated maize varieties show wide variation in their resistance to aphid feeding. Specific genes that mediate aphid resistance have been identified by quantitative trait locus (QTL) mapping of aphid progeny production on different maize inbred lines. To investigate the insect side of the interaction, Sugarcane mosaic virus, which infects maize and is transmitted by aphids, has been engineered for virus-induced silencing of aphid transcription. By reducing aphid gene expression in a targeted manner, it is possible to study the function of specific genes in plant-aphid interactions.
Arabidopsis-aphid interactions

A fourth-instar green peach aphid (Myzus persicae) on an Arabidopsis leaf
The green peach aphid (Myzus persicae) feeds readily on hundreds of plant species, including the genetic model plants Arabidopsis thaliana (Arabidopsis) and Nicotiana benthamiana (an Australian tobacco species). As broad generalist herbivores, green peach aphids are exposed to a wide variety of toxic metabolites in the plants from which they are feeding. Thus, green peach aphids must have broadly effective mechanisms to avoid or inactivate plant toxins and other defenses.
Analysis of salivary proteins, which are injected into the plant phloem when aphids are feeding, has demonstrated that some act as effectors that suppress plant defenses, whereas others are recognized by plants as signals to initiate defense responses. Silencing the expression of aphid salivary genes by RNA interference, in combination with Arabidopsis and N. benthamiana mutant lines, can demonstrate the specificity of such interactions. Ongoing research in the Jander lab is directed at identifying the functions of individual aphid salivary proteins, as well as their targeted interactions with defense signaling and metabolic pathways in plants.
Biosynthesis of cardiac glycosides in wallflowers and milkweeds

Wormseed wallflower (Erysimum cheiranthoides) in a growth chamber at the Boyce Thompson Institute.
Production of cardiac glycosides, plant defensive metabolites that are toxic to most animal species, has evolved several times in different plant families. However, despite hundreds of publications on the ecological functions and medical uses of cardiac glycosides, the complete biosynthetic pathway has not been identified any plant species. Working together with Tobias Züst at the University of Bern and other collaborators, the Jander lab has established Erysimum cheiranthoides (wormseed wallflower) as a new genetic and genomic model system for studying cardiac glycoside biosynthesis. Isolation of mutant lines, co-expression analysis, and comparative genomics have identified numerous candidate genes involved in cardiac glycoside biosynthesis by wallflowers. In addition to investigating the defensive functions of the different cardiac glycosides in wallflowers, a major goal of this research is to identify the complete biosynthetic pathway and thereby enable the production of cardiac glycosides in heterologous systems.
Like wallflowers, Asclepias syriaca (common milkweed) and Asclepias curassavica (tropical milkweed) produce cardiac glycosides as a defense against insect herbivory. Correlation of cardiac glycoside content and gene expression in different milkweed tissues has identified candidate genes for cardiac glycoside biosynthesis in milkweeds. The role of these genes in cardiac glycoside biosynthesis will be tested by expression silencing in common milkweed and/or tropical milkweed. Since wallflowers and milkweeds evolved cardiac glycoside biosynthesis independently, it is likely that they use similar but not identical biosynthetic pathways for the production of these important defensive metabolites.
-
Controlling insect pests by targeting genes acquired from other species
Killing crop-damaging insects by targeting genes essential to their survival is a promising approach to pest control. Because essential genes are often conserved across multiple insect species, the challenge is […] Read more » -
BTI Celebrates Another Year of Successful Summer Internship Programs
Boyce Thompson Institute celebrated its 22nd annual Plant Genome Research Program (PGRP) summer internship program with an award ceremony at the George and Helen Kohut Symposium, which was held at […] Read more » -
BTI Welcomes Summer Student Interns
On May 31, Boyce Thompson Institute welcomed 41 of the country’s brightest undergraduate students from universities around the country to experience the life of a researcher for 10 weeks. Ten […] Read more »
-
Congratulations to BTI’s Spring 2022 Graduates!
We are pleased to announce that three BTI researchers received their degrees during the Cornell University commencement ceremony on May 28. Congratulations to our newest alumni! Alex Ogbonna, Mueller lab, […] Read more » -
NSF Launches $25 Million Digital Biology Center
The National Science Foundation (NSF) awarded $25 million over five years to four participating institutions to create the Center for Research on Programmable Plant Systems (CROPPS), which will develop new […] Read more » -
BTI Graduate Students Receive Schmittau-Novak Grants
We would like to congratulate five BTI graduate students who are Spring 2020 Schmittau-Novak Grants Program recipients. Supported by a bequest from the estate of Jean Schmittau in honor of […] Read more » -
Wallflowers Could Lead to New Drugs
Plant-derived chemicals called cardenolides have long been used to treat heart disease, and have shown potential as cancer therapies. But the compounds are very toxic, making it difficult for doctors […] Read more » -
BTI Celebrates Another Successful Summer Internship Program
Boyce Thompson Institute celebrated its 19th annual Plant Genome Research Program (PGRP) summer internship program with an award ceremony at the George and Helen Kohut Symposium, which was held at […] Read more » -
Inaugural BTI Alumni Recognition Awards
It is with great enthusiasm and pride that Boyce Thompson Institute (BTI) will recognize the first recipients of BTI’s Alumni Recognition Awards during the 2019 PGS Career Symposium on April […] Read more » -
BTI scientists win awards at annual Northeast ASPB meeting
Two researchers from the Boyce Thompson Institute earned 1st place honors at the 2018 Northeast ASPB Section annual meeting. The meeting was hosted by the University of Massachusetts, Amherst, and the theme was Translational Research for Improving Crop Productivity. Read more » -
2017 PGRP Symposium marks finale of intern researchers’ summer
Now in its 16th year, BTI’s annual PGRP symposium provides a means for student interns to to present their findings in a professional, engaging setting. Read more » -
BTI Receives DARPA “Insect Allies” Award to Develop Viruses and Insects for Maize Improvement
The research project, titled Viruses and Insects as Plant Enhancement Resources (VIPER), is supported by the Defense Advanced Research Projects Agency (DARPA) Insect Allies program. Read more » -
Georg Jander receives $1 million NSF EDGE award to develop genomic tools for studying milkweed
BTI’s Georg Jander is leading one of eight research groups selected to receive awards through the Enabling Discovery through Genomic Tools (EDGE) program, overseen by the National Science Foundation’s (NSF’s) Biological Science Directorate. Read more » -
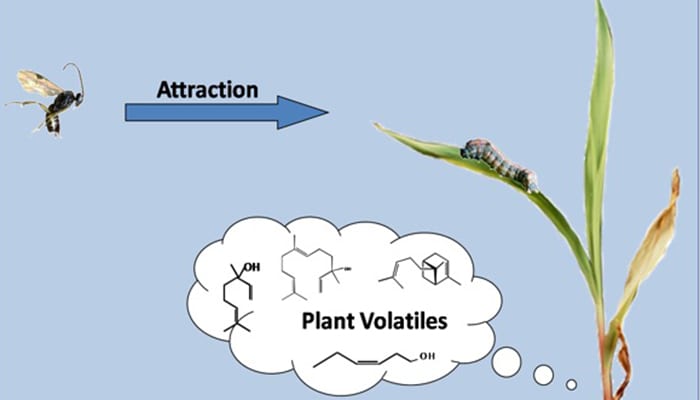
Researchers Identify Genes for “Help Me!” Aromas from Corn
Insect damage triggers volatile compounds that attract caterpillar-killing wasps. Read more » -
Jander Lab Members Win ASPB Awards
Postdoctoral researcher Vered Tzin received support to present her work at the American Society for Plant Biology annual meeting in July, and Cairo Archer received an undergraduate research fellowship to support her summer research in the Jander lab Read more » -
Potato Plants Trigger Aboveground Defenses in Response to Tuber Attacks
Mimicking the effects of a Guatemalan tuber moth infestation in agricultural fields could increase potato yield and reduce pest damage. Read more » -
BTI Hosts Visit from My Brother’s Keeper Group
The group from Team I.M.P.A.C.T. of Rochester, NY, exposed young men to potential careers in the plant sciences. Read more » -
The Herbivores Dilemma
Jander lab members investigate the chemical defenses that young corn plants use to fight off simultaneous attacks from hungry insects. Read more » -
Aphids Balance Their Diets by Rebuilding Plant Amino Acids
Aphids thrive on a high-sugar diet, thanks to bacterial partners that help them breakdown plant sap and build essential amino acids from scratch. Read more » -
BTI Scientists Envision the “Future of Food”
What will your dinner plate look like in 2050? With discoveries from the Boyce Thompson Institute, future crops may have more nutrients and greater resistance to insects, drought and disease. Read more » -
Cairo Archer Wants to Level the Growing Field
“There is such a disconnect between what the average person knows about plant science and what we do in the lab here...I think it’s really important to be able to talk to anyone about what I do in a way that they understand.” Read more » -
Teachers Become Students at BTI’s Curriculum Development Projects
Fourteen teachers arrived at BTI from schools as close as Ithaca and as far as Anaheim, Calif. to attend the BTI Plant Biology Curriculum Development Projects (CDP) Teacher Institute July 13-17, 2015 Read more » -
Teachers Share Strategies for Inspiring Tomorrow’s STEM Researchers
Science teachers planted switchgrass seeds, sampled algae-glycerin soap, and participated in roleplaying activities at the Bioenergy and Bioproducts Education Program’s National STEM conference last week in Horseheads, N.Y. Read more » -
Felix Fernandez-Penny Stays True to His Roots
Some interns, like Cornell University first-year student Felix Fernandez-Penny, enjoy their time at BTI so much that they keep showing up at the laboratory, long after the summer ends. Read more » -
BTI Welcomes New Crop of Summer Interns
BTI welcomes 20 college-level interns for 10 weeks of research in Plant Genome Research Program, the Bioinformatics Program or the Bioenergy Education Program. Read more » -
Professor Jander Comments on Citrus Greening Research
EPA has granted temporary approval of two genes from spinach to be used in citrus plants. "There is a critical need to go beyond citrus to find novel resistance genes that provide protection..." Read more » -
Why Rice Can’t Get Along with Its Neighbors
A new compound could make rice more weed-resistant. Researchers in the Jander laboratory first discovered b-tyrosine while looking for new defense compounds in rice. Read more » -
New equipment opens up ‘mass’ive possibilities
The Boyce Thompson Institute starts off 2015 with a generous gift from the Triad Foundation and researchers are about to open their most exciting present: a high-resolution mass spectrometer. The […] Read more » -
Jander Unlocks Molecular Pathways of Plants and Empowers People
Professor Georg Jander has seen that, when attacked, plants react at the molecular level. Jander’s dedication—to plant science and people—seems to be contagious. Read more » -
Hemiptera Conference Held at Cornell, December 2014
Georg Jander, Michelle Cilia and Angela Douglas organized Hemiptera (sucking insects) conference held on December 4, 2014. Read more » -
New Website Is Portal for Nicotiana benthamiana Experimental Resources
This Nicotiana benthamiana web site shares papers, results, tools, protocols, and other materials from researchers using NB as a study plant. Read more » -
Three Boyce Thompson Institute Scientists Honored as AAAS Fellows
Drs. Harrison, Klessig, and Jander honored. Read more » -
Sugar Could Be a Sweet Way to Control Insect Pests
BTI Scientist Georg Jander to work with Cornell University’s Angela Douglas to study the effects of sugar on insects, to control pests on plants. Read more »
Plants in nature are subject to attack by wide variety of caterpillars, beetles, aphids, and other insect herbivores. Although there are a million or more species of herbivorous insects, any individual plant species is resistant to the vast majority of these. Insect feeding is inhibited by an array of chemical defenses that exhibits great variability both within and among different plant species. However, although it is known that any plant leaf contains several thousand different metabolites, most of these remain unidentified. In the Jander lab we are investigating natural variation in the herbivore resistance of maize, tomato, and potato to elucidate the molecular basis of plant defense traits. Through a combination of genetic crosses, gene expression assays, metabolite profiling, and insect growth experiments, we are able to identify specific plant genes, biosynthetic pathways, and metabolites that are required to mount an effective anti-herbivore defense.
Internship Program | Projects & Faculty | Apply for an Internship