Please see our Group Website for recent news and publications, research updates, and teaching.
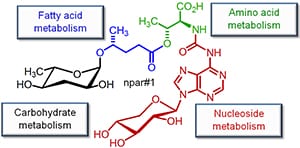
Our research is directed at characterizing structures and biological functions of biogenic small molecules (BSM’s). BSM’s play important roles in most biological processes, and detailed knowledge of their chemical structures and their interactions with other biomolecules is essential for advancing our molecular understanding of life. BSM’s regulate development and immune responses in plants and animals, and serve important functions in interactions of different organisms with each other. As a result, an organism’s metabolome essentially comprises a collection of small molecules with potentially useful affinities for specific molecular targets. Not surprisingly, BSM’s constitute the most important source of lead structures for drug development.
Compared to template-derived biological macromolecules such as proteins and nucleic acids, BSM’s are chemically much more diverse and correspondingly present great analytical challenges. As a result, genomic and proteomic knowledge has not yet been complemented by a comprehensive characterization of structures and functions of metabolomes, presenting one of the most significant barriers toward advancing our understanding of biological pathways.
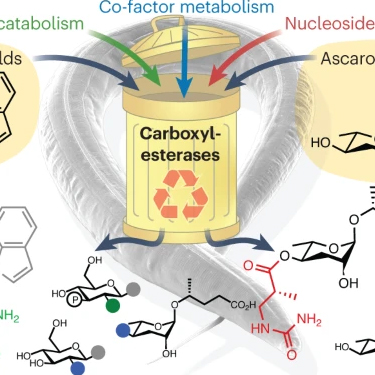
The Schroeder lab aims to help close this knowledge gap by developing approaches for a more systematic structural and functional characterization of BSM’s. Usually, BSM’s occur as – often minor – components of a more or less complex biological matrix, comprising a large number of BSM’s and other biomolecules. Traditional approaches for the characterization of BSM’s such as HPLC-MS or activity-guided fractionation have distinct disadvantages that severely limit their applicability. Our aims is to develop NMR spectroscopy-based approaches that complement or enhance traditional methodology by enabling detailed characterization of BSM’s in complex biological samples, with regard to both chemical structure and biological function.
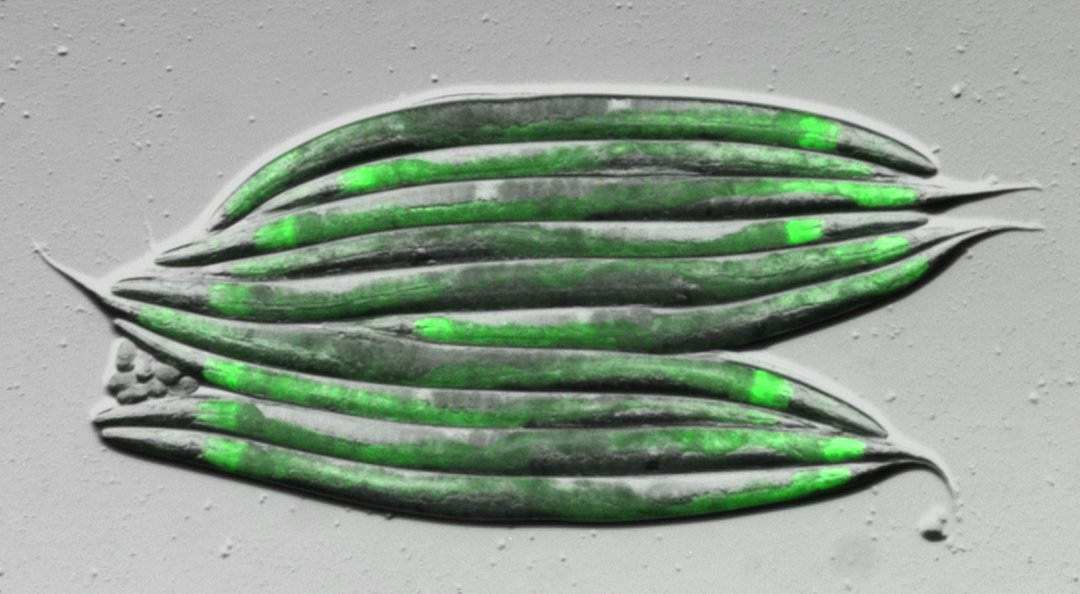
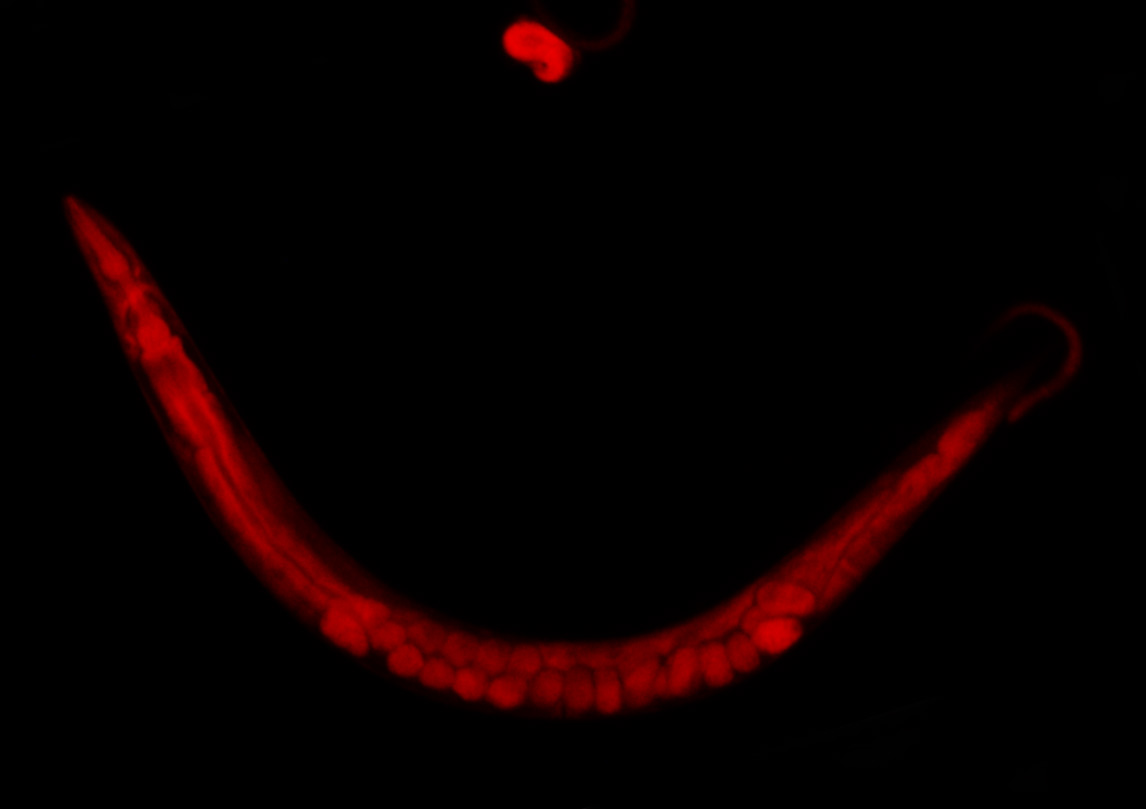
Based on NMR-spectroscopic methodology we have engaged in a comprehensive effort to characterize structures and functions of the metabolome (the entirety of all BSM’s) produced by the model organism Caenorhabditis elegans, focusing on several newly discovered compounds that control development, and ultimately lifespan. In addition we have started a project directed at investigating the chemical ecology of microorganisms in search of leads for new antibiotics. Complementing our interests in analytical chemistry, we pursue development of efficient syntheses for newly identified compounds with particular biological significance.
Please visit our research pages for more details!
-
New insights into how serotonin regulates behavior
Rates of anxiety and depression have been increasing around the world for decades, a trend that has been sharply exacerbated by the COVID-19 pandemic. New research led by the Boyce […] Read more »
-
Worms as a model for personalized medicine
Tailoring a person’s diet or medicine based on their genomes has been a goal of the medical community for decades, but the strategy has not been widely successful because people […] Read more » -
New software to help discover valuable compounds
As a postdoctoral research associate in the lab of BTI faculty member Frank Schroeder, Max Helf saw his labmates continually struggle when they were analyzing data. So, he decided to […] Read more » -
Congratulations Spring 2020 Graduates!
We are pleased to announce that six BTI researchers received their degrees from Cornell University this spring. Congratulations to our newest alumni: Jason Hoki, Schroeder lab, PhD in Chemistry & […] Read more » -
Aspirin-Like Compounds Could Treat Numerous Human Diseases
People have used aspirin to treat pain, fever and inflammation for more than a century, and the drug is also used to reduce the risk of strokes, heart attacks and […] Read more » -
BTI Researchers Publish High-Impact Nature Papers
We would like to congratulate a pair of BTI faculty members who recently published high-impact research papers in the prestigious research journal Nature. Frank Schroeder and colleagues discovered the first […] Read more » -
BTI Researchers Discover Compound that Speeds Sexual Development and Decline
Every day, people are exposed to myriad chemicals, both natural and synthetic. Some of these compounds may affect human physical development, but testing them directly on people would be grossly […] Read more » -
Worm Pheromones Protect Major Crops
Protecting crops from pests and pathogens without using toxic pesticides has been a longtime goal of farmers. Researchers at Boyce Thompson Institute have found that compounds from an unlikely source […] Read more » -
Congratulations to BTI’s PhD Graduates!
We are pleased to announce that seven Boyce Thompson Institute researchers received their PhD degrees during the Cornell University commencement ceremony on May 26. Congratulations to our newest alumni: Mariko […] Read more » -
Inaugural BTI Alumni Recognition Awards
It is with great enthusiasm and pride that Boyce Thompson Institute (BTI) will recognize the first recipients of BTI’s Alumni Recognition Awards during the 2019 PGS Career Symposium on April […] Read more » -
Ascribe Bioscience Receives SBIR Award from NSF
On January 29, 2019, Ascribe Bioscience became the first company based on technology developed at the Boyce Thompson Institute (BTI) to receive a Small Business Innovation Research (SBIR) grant. The […] Read more » -
BTI promotes faculty members Schroeder and Van Eck
Boyce Thompson Institute president, David Stern, has officially announced promotions for faculty members Frank Schroeder and Joyce Van Eck. Both researchers were thoroughly reviewed and evaluated on both their achievements to date and the potential they possess. Read more » -
$9.4M NIH grant funds chronic fatigue syndrome center
Cornell will receive close to $9.4 million over five years to establish the Cornell Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Collaborative Research Center, which will span Cornell’s Ithaca campus, Weill Cornell Medicine, Ithaca College, the Boyce Thompson Institute [Schroeder Lab], the Workwell Foundation, EVMED Research, the SOLVE ME/CFS Initiative and private ME/CFS medical practices. Read more » -
Frank Schroeder Selected for HHMI Faculty Scholars Program
The five-year grant is given to innovative, early career scientists to support high-risk research with the potential to make significant contributions to the field. Read more » -
Researchers Receive $1.7M NIH Grant for Nematode Behavior Work
When C. elegans larvae face starvation, they clump together in a mass of worms, which increases their lifespan. BTI researchers will explore this fascinating social behavior. Read more » -
Chemicals from Parasitic Worms Boost Plant Immunity
When plants detect pheromones given off by nematode worms, they activate their immune system for protection. The chemical warning not only triggers defenses against nematodes, but also against bacterial, fungal and viral infection. Read more » -
BTI Hosts Flash Science! Speaking Competition
BTI Professor Emeritus Robert Kohut initiates competition at BTI to give early-career scientists an opportunity to communicate with the general public and practice their “elevator speech.” Read more » -
New equipment opens up ‘mass’ive possibilities
The Boyce Thompson Institute starts off 2015 with a generous gift from the Triad Foundation and researchers are about to open their most exciting present: a high-resolution mass spectrometer. The […] Read more » -
Patrick Boyle Off to Private Sector
Dr. Patrick Boyle will be an "Emerging Leader in Science" after four years as a Postdoctoral Scientist in Professor Gregory Martin’s Lab at BTI. Read more » -
BTI/MIT Awarded Transformative NIH Grant to Tackle Antibiotic “Discovery Void”
$3 Million cross-cutting interdisciplinary High-Risk, High-Reward research project taps into unexplored source of antimicrobial compounds. Read more » -
Postgraduates Hosted Annual BTI Symposium, September 25, 2014
Speakers will include Dr. Frank Schroeder, postdocs Daniela Floss and Patricia Manosalva, Dr. Robert Granados and Dr. Maureen Hanson (from Cornell), and discussion of postgraduate training and education with Dr. David Stern and Dr. Eric Richards. Read more » -
Schroeder Lab: Answering Life’s Biggest Questions with Small Molecules
Schroeder's lab works to discover new small molecules and identify their structure and function in the context of aging. “We know everything we see will be unknown, pure discovery...It's pretty intense. We get to the bottom of things.” Read more » -
Joshua Judkins, BTI and Beyond
Joshua Judkins arrived at BTI as an intern in 2008 and left as a PhD in 2014...for a post doc position with Pfizer in neuroscience. Read more » -
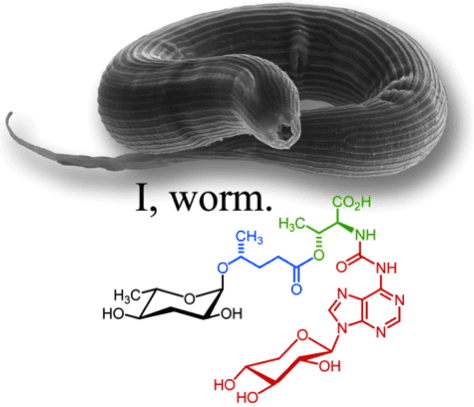
Worms Hijack RNA Building Blocks, Create Unique Sugar in the Process
Conventional wisdom holds that genes determine the morphology of animals, but something else iwormmay be at play. Frank Schroeder from the Boyce Thompson Institute at Cornell University and Ralf Sommer from the Max-Planck Institute in Germany now rep. Read more » -
Compounds in Worms May Lead to Parasite Treatment
All of these nematodes speak the same chemical language,” through the use of compounds called ascarosides, said study co-author Frank Schroeder. Read more » -
Worms Communicate Using a Complex Chemical Language
Scientists have discovered that a species of small, transparent roundworms called Caenorhabditis elegans possess a highly-evolved language in which they combine chemical fragments to create precise molecular messages. Read more » -
Frank Schroeder Published in Nature: Sex and Life Span Linked in Worms: A Family of Sugar
A group of scientists who set out to study sex pheromones in a tiny worm found that the same family of pheromones also controls a stage in the worms’ life cycle, the long-lived dauer larva. Read more »