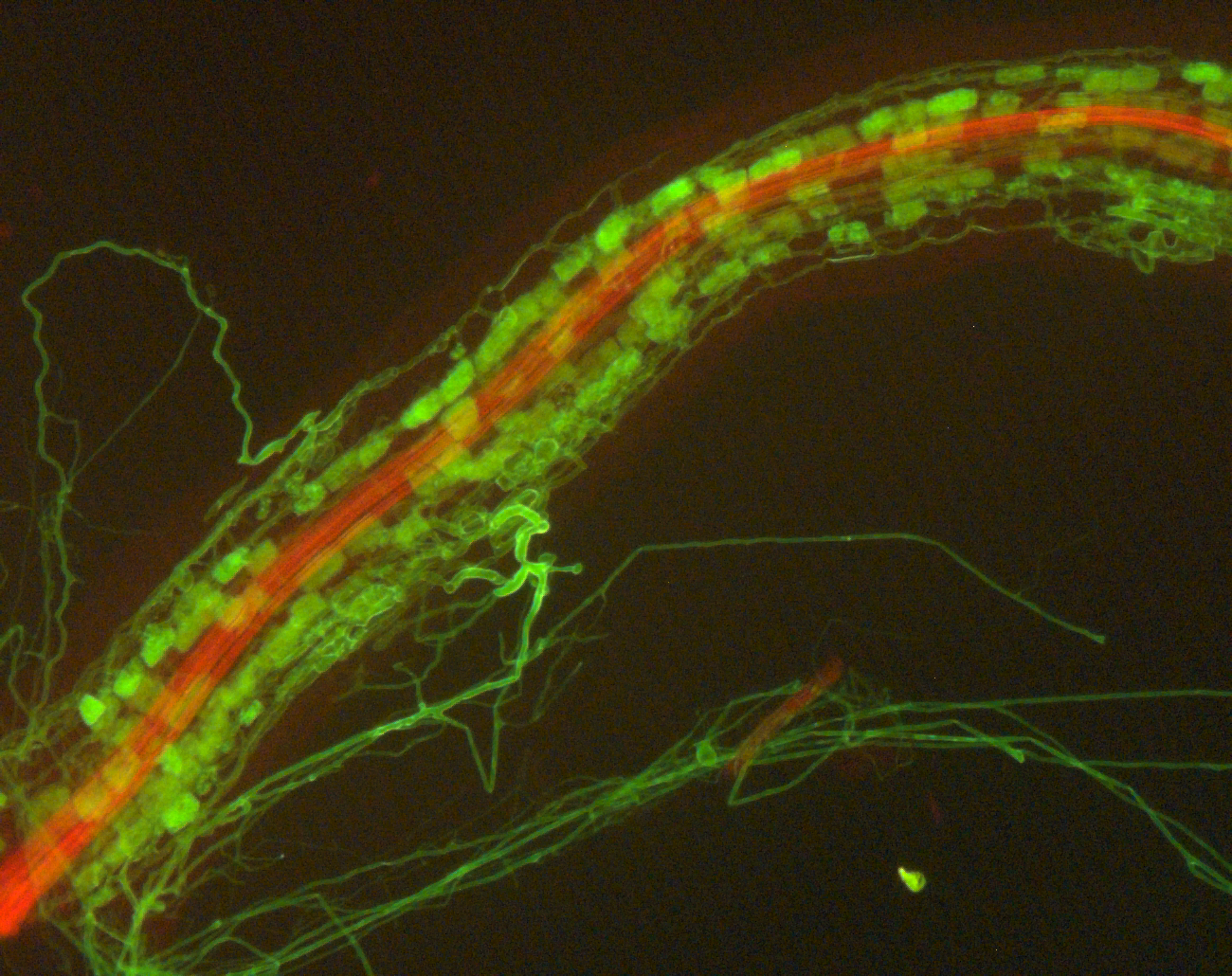
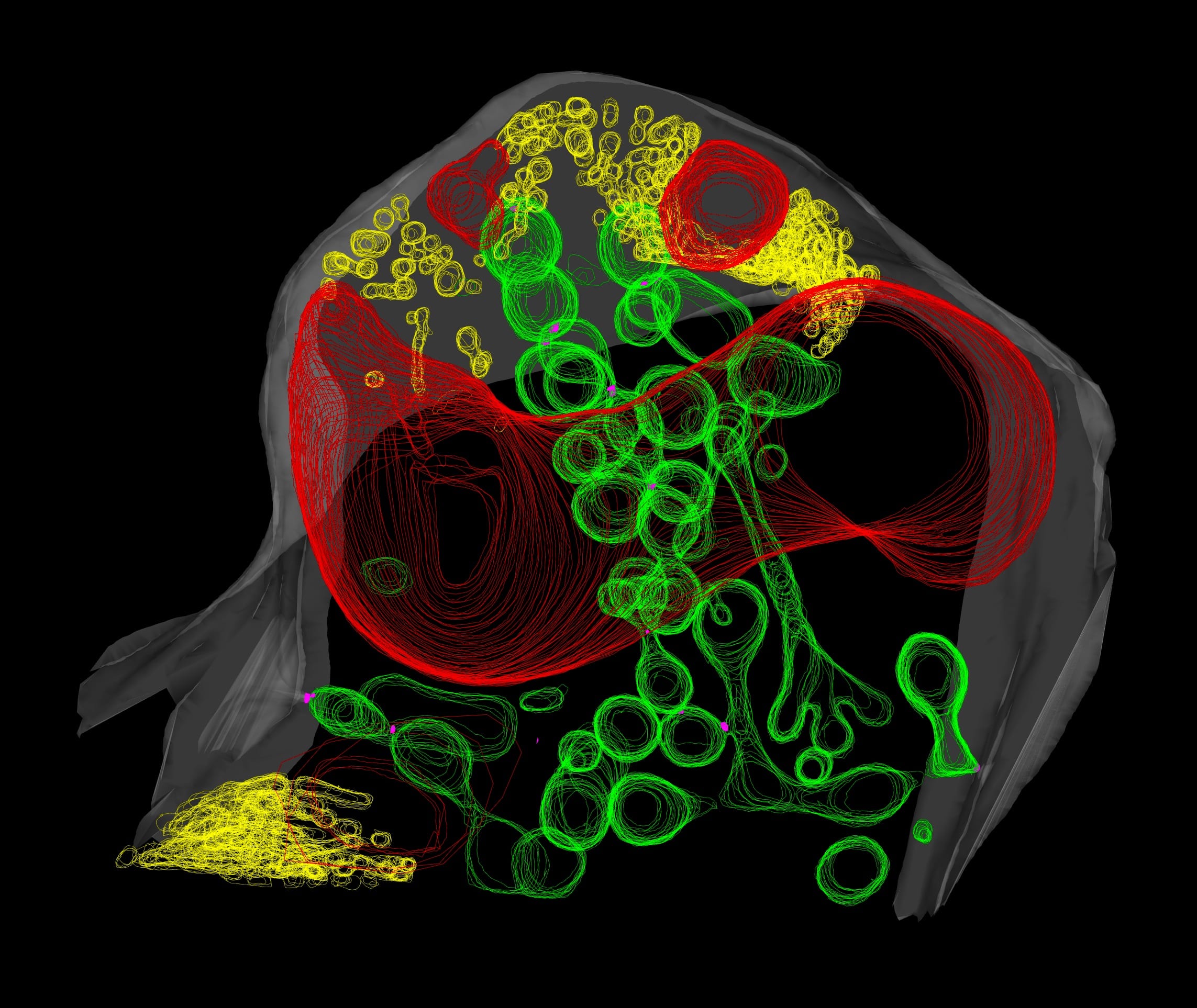
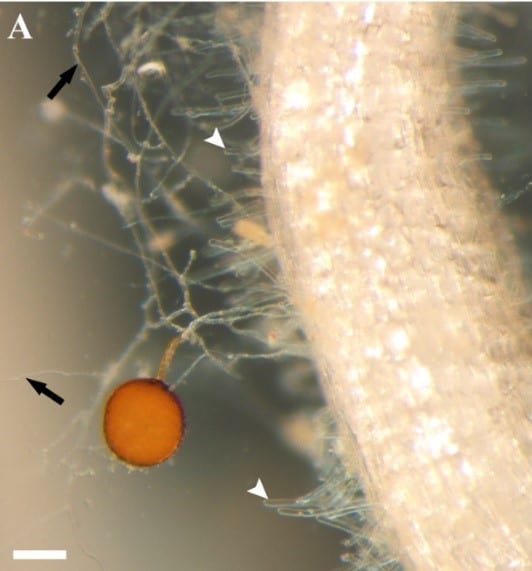
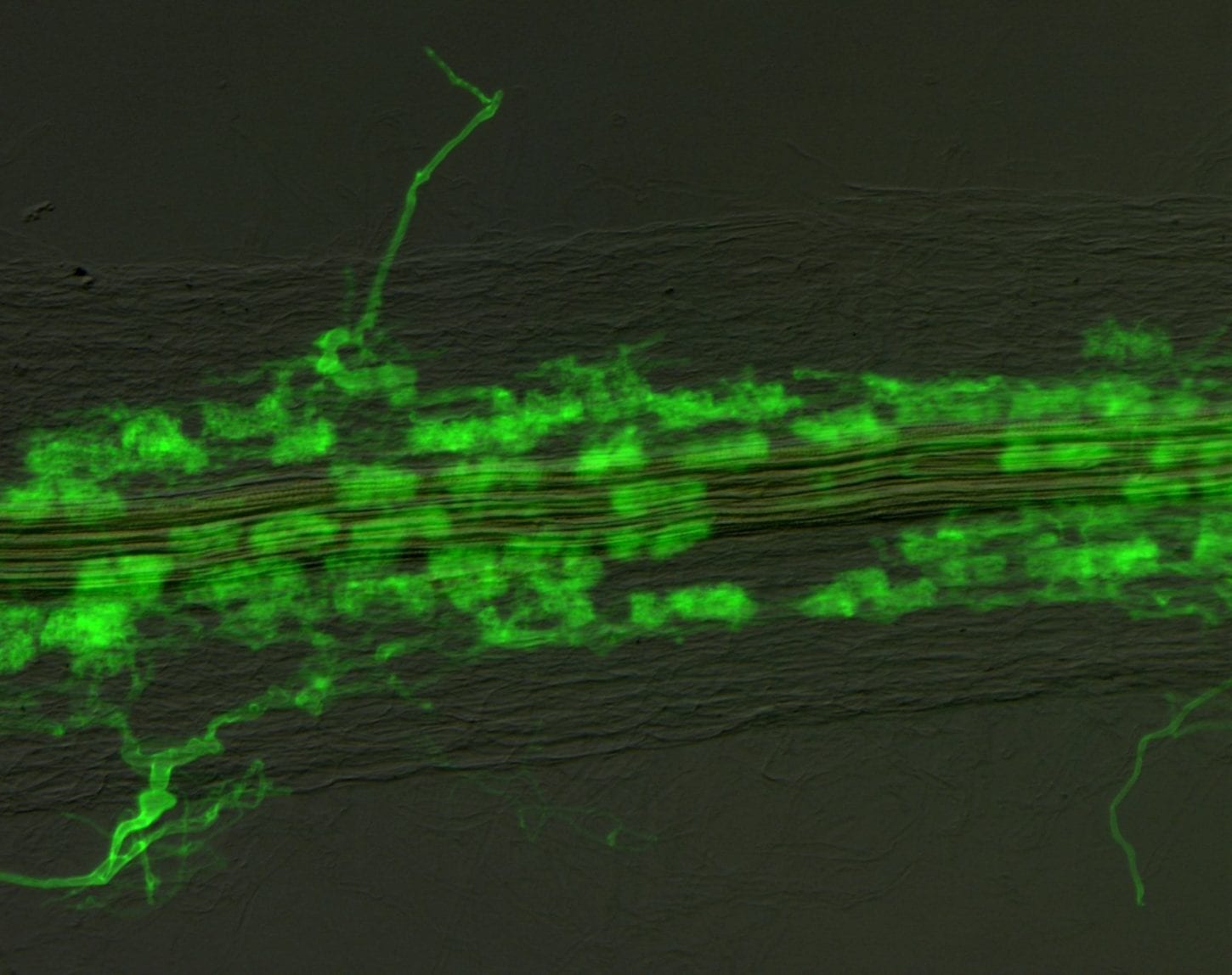
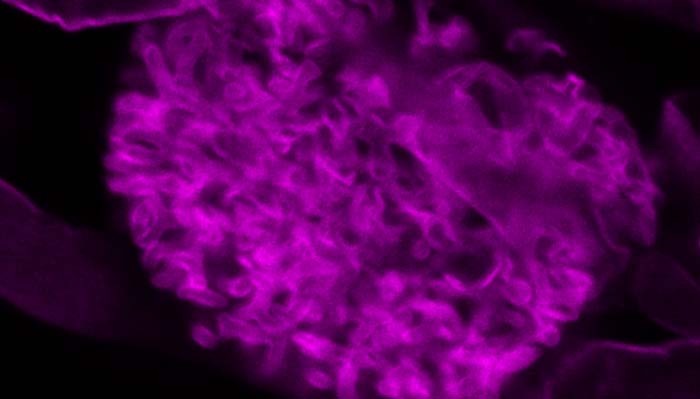
Most vascular flowering plants are able to form symbiotic associations with arbuscular mycorrhizal (AM) fungi. These associations, named ‘arbuscular mycorrhizas’, develop in the roots, where the fungus colonizes the cortex to access carbon supplied by the plant. The fungal contribution to the symbiosis includes the transfer of mineral nutrients, particularly phosphorus, from the soil to the plant. In many soils, phosphate exists at levels that are limiting for plant growth. Consequently, additional phosphate supplied via AM fungi can have a significant impact on plant development, and this symbiosis influences the structure of plant communities in ecosystems worldwide.
The long-term goals of our research are to understand the mechanisms underlying development of the AM symbiosis and phosphate transfer between the symbionts. A model legume, Medicago truncatula, and arbuscular mycorrhizal fungi, Glomus versiforme, Glomus intraradices and Gigaspora gigantea are used for these analyses. Currently, a combination of molecular, cell biology, genetic and genomics approaches are being used to obtain insights into development of the symbiosis, communication between the plant and fungal symbionts, and symbiotic phosphate transport.
Current open position:
Postdoctoral Associate: Arbuscular mycorrhizal symbiosis and hyphae-associated microbial communities
-
Phosphate biosensors could lead to more efficient fertilizer usage
Shiqi Zhang spent many months sitting alone in a dark room, staring intently into the lens of a confocal microscope as she focused a laser beam on plant cells mounted […] Read more » -
BTI Welcomes Summer Student Interns
On May 31, Boyce Thompson Institute welcomed 41 of the country’s brightest undergraduate students from universities around the country to experience the life of a researcher for 10 weeks. Ten […] Read more »
-
Fungi could manipulate bacteria to enrich soil with nutrients
A team of researchers from the Boyce Thompson Institute (BTI) has discovered a distinct group of bacteria that may help fungi and plants acquire soil nutrients. The findings could point […] Read more » -
Congratulations Spring 2020 Graduates!
We are pleased to announce that six BTI researchers received their degrees from Cornell University this spring. Congratulations to our newest alumni: Jason Hoki, Schroeder lab, PhD in Chemistry & […] Read more » -
Plant Gene Discovery Could Help Reduce Fertilizer Pollution in Waterways
Over-fertilization of agricultural fields is a huge environmental problem. Excess phosphorus from fertilized cropland frequently finds its way into nearby rivers and lakes. A resulting boom of aquatic plant growth […] Read more » -
Congratulations to BTI’s PhD Graduates!
We are pleased to announce that seven Boyce Thompson Institute researchers received their PhD degrees during the Cornell University commencement ceremony on May 26. Congratulations to our newest alumni: Mariko […] Read more » -
BTI’s Maria Harrison Elected to National Academy of Sciences
Maria Harrison, William H. Crocker Professor at Boyce Thompson Institute and Adjunct Professor in the School of Integrative Plant Science (SIPS) at Cornell University, has been elected to the National […] Read more » -
Plant–Fungal Interface Gets Tubular
For hundreds of millions of years, plants and fungi have formed symbiotic relationships to trade crucial nutrients, such as phosphate and fatty acids. This relationship is extremely important to the […] Read more » -
Back to our roots: Insights from genomes of a plant-associated fungus and its bacterial endosymbionts
In an article published this month in the journal New Phytologist, researchers at the Boyce Thompson Institute and the National Center for Genome Resources describe the genome sequences (DNA sequences), […] Read more » -
Maria Harrison featured in interview with International Society for Molecular Plant-Microbe Interactions
In this IS-MPMI's interview with Kevin Cope (University of Wisconsin-Madison), Maria Harrison discusses her research on arbuscular mycorrhizal fungi and provides advice to young scientists entering the field. Read more » -
Maria Harrison, consortium of scientists receive $5 million grant to study genes that help legumes access soil nutrients
BTI's Harrison lab will develop Medicago truncatula mutants to identify the function of genes predicted to be important in nitrogen fixation in legumes. Read more » -
400 million years of a stable relationship: clues to the molecular basis of balance in AM symbiosis
Researchers from the Harrison lab at BTI have identified a transcriptional program that drives arbuscule degeneration during AM symbiosis. This regulation of arbuscule lifespan has likely contributed to the 400MY stability of the symbiosis by preventing the persistence of fungal cheaters. Read more » -
Feeding fat to fungi: evidence for lipid transfer in arbuscular mycorrhiza
Researchers from the labs of Dr. Maria Harrison at the Boyce Thompson Institute and Dr. Peter Dörmann at the University of Bonn have produced the first experimental evidence to suggest that AM fungi also get lipids from the plant. AM-induced FatM and RAM2 may play specific roles in the biosynthesis of 16:0 βMAG, which cannot be produced by the fungus, providing a clue to understanding the obligate nature of AM fungi. Read more » -
‘New Visions’ of food security from Cassandra Proctor
“Food security is a mixture of all the different aspects of agriculture. It’s not just growing the food," said Proctor. “It’s not just planting something in the ground – there is a lot more to it.” Read more » -
Researchers Uncover Core Set of Genes for Plant-Fungal Symbiosis
BTI researchers used a genome comparison approach to identify genes necessary for beneficial plant-fungal relationships, which may lead to better crop plants that require less fertilizer input. Read more » -
Harrison Receives Grant for Phosphate Biosensor Research
Fluorescent proteins will allow researchers to track phosphate movement through cells in real time. Read more » -
Better Biofuels: Harrison Collaborates on Sorghum Project
Maria Harrison will participate in a $13.5 million, multi-institution systems biology project with Daniel Schachtman of the University of Nebraska-Lincoln to develop sorghum that is more drought resistant and uses nitrogen more efficiently. Read more » -
Scientists Unravel Root Cause of Plant Twists and Turns
BTI researchers Harrison and Floss collaborate with Cornell physicists to understand how roots grow around barriers in the soil, while still heading down. Read more » -
Key Protein for Plant-Fungal Symbiosis Discovered
Harrison Lab has discovered that plants use EXO70I to form a membrane around the fungus in arbuscular mycorrhizal (AM) symbioses, beneficial associations where plants receive phosphate from fungi in exchange for carbohydrates. Read more » -
BTI Hosts Flash Science! Speaking Competition
BTI Professor Emeritus Robert Kohut initiates competition at BTI to give early-career scientists an opportunity to communicate with the general public and practice their “elevator speech.” Read more » -
Planting a Passion for Research
Marshall Tyler nominated Maria Harrison for the 2015 Cornell University College of Agriculture and Life Sciences Faculty Excellence in Undergraduate Research Mentoring Award. Read more » -
Ammonium Transporter Saves Plant-Fungal Symbiosis, When Nutrients Run Low
The discovery of the role of this ammonium transporter improves the understanding of how the plant and fungal partners regulate the symbiosis and how phosphate and nitrogen move through the system. Read more » -
Maria Harrison Wins ASPB’s Dennis R. Hoagland Award
Professor Maria Harrison is the 2015 winner of the Dennis R. Hoagland Award from the American Society of Plant Biologists, given every three years, for her outstanding work on plant mineral nutrition. Read more » -
Eaglesham Bridges BTI and Both Cambridges
James Eaglesham began his career as an intern at BTI, and heads to the University of Cambridge, England as a Churchill scholar for pathology research. When back in US, Eaglesham will pursue his doctorate in virology at Harvard, in Cambridge, MA. Read more » -
BTI Scientist Maria Harrison is Co-principal Investigator on $6.5M NSF Award
The research project will study genes responsible for beneficial symbioses with bacteria and fungi. Work in the Harrison lab will focus on the plant-fungal AM symbiosis and how it improves phosphorus uptake in plants. Read more » -
Maria Harrison Named William H. Crocker Scientist
At its May meeting, the BTI Board of Directors named Maria Harrison as the William H. Crocker Scientist. Read more »
Phosphorus is a critical macronutrient for proper plant growth. While phosphorus deficiencies can be improved by the application of phosphate fertilizers, it is costly, both to the farmer and to the environment. Furthermore, the crops only take up a small percentage of the applied fertilizer; the remainder is either immobilized in the soil, or carried into ground water and rivers, often resulting in pollution.
Interns in the Harrison lab investigate two aspects of plant phosphorus nutrition. The first aspect seeks to understand the basis for the symbiotic relationships between vascular flowering plants and arbuscular mycorrhizal (AM) fungi. The fungi colonize root cells, gaining access to carbon supplied by the plant, while at the same time mobilizing mineral nutrients from the soil, including phosphorus, to be used by the plant. For this work, the lab uses the model legume, Medicago truncatula and the fungus Glomus versiforme. The Harrison lab also studies how plants find and take up phosphorus from the soil when they do not have these symbiotic relationships with fungi. This work toward understanding the mechanisms of perception and acquisition of phosphorus by plants may eventually lead to a more effective usage of fertilizers.
Internship Program | Projects & Faculty | Apply for an Internship
Recent Publications (free access)
Receptor-associated kinases control the lipid provisioning program in plant–fungal symbiosis
Presentations for Broad Audience